Physical and chemical changes virtual lab. The chemical change is phosphoric acid in the soda pop reacting with calcium in the milk to make two new products.

Select one of the four events and view the video.
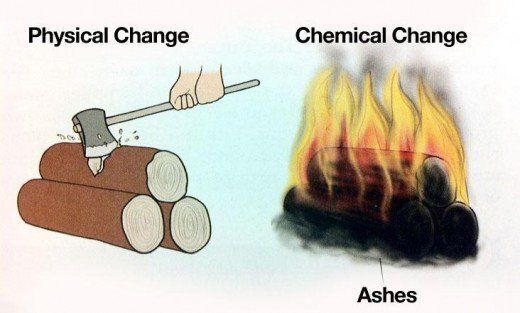
Physical and chemical changes vital lab. Physical And Chemical Changes Vital Lab Background when a physical change occurs only the form of the substance changes. Chemical changes however result in the formation of new substances with different properties. Some general signs of a chemical change include a change of color or odor the formation of a precipitate solid the formation of a gas and a change in heat or light.
In a physical change matter changes its appearance physical properties but not its composition. Examples of physical changes include changes of state between solid liquid and gas phases and making mixtures. On the other hand in a chemical change matter does change its composition new substances are made.
Physical and chemical changes virtual lab. Abrupt end of the last ice age approximately 11700 years ago by marking the beginning of the modern era climate A and human civilization. Most of these climatic changes are assigned to small variations in the eartha s that.
Physical and Chemical Changes Virtual Lab Procedure. Select one of the four events and view the video. You can stop the video at any point and watch it as many times as you need to.
Click the PlayPause rewind fast forward button when needed. Use your observations to check all the items on the Observations Checklist on the screen. Describe matter based on its physical and chemical properties.
Prove understanding of the difference between physical and chemical properties by sorting matter. Chemical Physical Changes Virtual Lab activity WEBSITE 1 httpvitalcsohiouedusteamwebsitedownloadsChangeLabswf 1. Complete the chart with the appropriate information.
Example Chemical or Physical Change Reason Glass Fireworks Water Freezing Photosynthesis Snowman. In this Virtual Lab you will view videos of matter changing and determine whether the changes you observe are physical or chemical in nature. Identify characteristics of physical changes of matter Identify characteristics of chemical changes of matter Recognize examples of physical and chemical changes.
Chemical and Physical Change Lab. Identify whether these lab experiments are demonstrating a physical change or a chemical change. Many ADA accommodations have proven very beneficial to many able-bodied workers such as persons shorter or taller than the average 510 American male workers who are aging andor exhibit a wide range of physical and sensory difficulties and women who are pregnant.
All these find laboratory-working conditions more useful and safe 2. Normal Values for Lab Tests. Lab tests along with a health history and physical exam are used by physicians to diagnose and manage health conditions.
When your body is healthy it functions normally in a state of homeostasis or equilibrium. When your body is in homeostasis the values for fluids chemicals electrolytes and secretions. For example melting ice tearing a sheet of paper into strips and dissolving sugar in water are physical changes that dont change the chemical identity of matter.
Here are some signs of a chemical reaction. If more than one sign is present its like a reaction has occurred. Bubbling or gas production.
The surprising chemical change occurs throughout the bottle and thats followed by a physical change as the curdled milk slowly falls to the bottom. The chemical change is phosphoric acid in the soda pop reacting with calcium in the milk to make two new products. Tricalcium phosphate and hydrogen.
Chemical and Physical Change Lab. Identify whether these lab experiments are demonstrating a physical change or a chemical change. Physical and Chemical Changes.
Lab 2 PHYSICAL SEPARATION TECHNIQUES Introduction When two or more substances that do not react chemically are blended together the result is a mixture in which each component retains its individual identity and properties. The separation of the components of a mixture is a problem frequently encountered in chemistry. A biology resource site for teachers and students which includes lesson plans student handouts powerpoint presentations and laboratory investigations.
The concept of change management in the laboratory environment varies markedly from methods typically prescribed for example in manufacturing operations. A physical chemical inventory should be performed at least annually or as requested by the CHO. Safety training should be viewed as a vital component of the laboratory safety.
Chemistry happens in the world around you not just in a lab. Matter interacts to form new products through a process called a chemical reaction or chemical change. Every time you cook or clean its chemistry in action.
Your body lives and grows thanks to chemical reactions. There are reactions when you take medications light a match and draw a breath. Physical changes are usually about physical states of matter.
Chemical changes happen on a molecular level when you have two or more molecules that interact. Chemical changes happen when atomic bonds are broken or created during chemical reactions. No Change to Molecules When you step on a can and crush it you have forced a physical change.
Chemical reactions are how new forms of matter are made. While nuclear reactions also may produce new matter nearly all the substances you encounter in daily life are the result of chemical changes. Chemical reactions help us understand the properties of matter.
By studying the way a sample interacts with other matter we can learn its chemical properties. The below page is useful for pre-lab in-lab and post-lab activities. A substance can be identified by observing its chemical and physical properties.
Physical properties are those that a substance can exhibit without undergoing a change in chemical composition. Regular blood testing is one of the most important ways to keep track of your overall physical well-being. Getting tested at routine intervals can allow you to see the way your body changes over.
As the names suggest a physical change affects a substances physical properties and a chemical change affects its chemical properties. Many physical changes are reversible such as heating and cooling whereas chemical changes are often irreversible or only. Determine if each is a physical or chemical change.
This activity was created by a Quia Web subscriber.