HYDROLYSIS OF SALTS AND pH OF BUFFER SOLUTIONS REPORT SHEET TABLE 75 Solution Ion Expected to Hydrolyze None Spectator Ion Naa C2H302 Nort 010 M Naci 010 M Naco 010 M NaCHO 010 M NHẠC 010 M ZnCI 010 M KAlSO 2n Zn24 So 4²- TABLE 76 Methyl Solution Red Bromothyol Blue Methyl Orange Phenol Red Phenoph- thalein Alizarin Yellow pH H OH 1 HO 883. The pH of a salt composed of weak acid and weak base will depend on the strength relationship of the acidic and basic components of the salt.
Salt of a strong acid and a strong base.
Hydrolysis of salts and ph of buffer solutions. The first part part A is about pH solution and hydrolysis of salt and part B is about the pH of buffer solution. In part A students will start with unboiled water and place the water in 5 separate test tubes and a few drops of pH indicator will be put into the test tubes. View full document.
Dipaly Bhakta 04202016 Hydrolysis of Salts and pH of Buffer Solutions Abstract The objective of this experiment was to determine the value of pH of salt and solution by using hydrolysis and buffer method respectively Nelson 2015. We determined the pH values of salt solutions by using different indicators calculated the Ka. Epxriment is divided into two parts.
The first part part A is about pH solution and hydrolysis of. Salt and part B is about the pH of buffer solution. In part A students will start with unboiled.
Water and place the water in 5 separate test tubes and a few drops of pH indicator will be put into. TITLE Hydrolysis of salt and pH of buffer solutions. ABSTRACT This experiment has four objective to be achieved that are to determine pH values of salts solutions by using different indicators to calculate the Ka or Kb for each cation or anion that hydrolyzes preparing an acetic acid-sodium acetate buffer and to investigate the effect of acid on the buffer pH.
Luis Molina 42620 Hydrolysis of Salt and pH of Buffer Solutions report I. Introduction I will expect solutions of substances such as HCl and HNO2 to be acidic and solutions of NaOH and NH3 to be basic. However I may be somewhat surprised to discover that aqueous solutions of some salts for example sodium nitrate NaNO2 and potassium acetate.
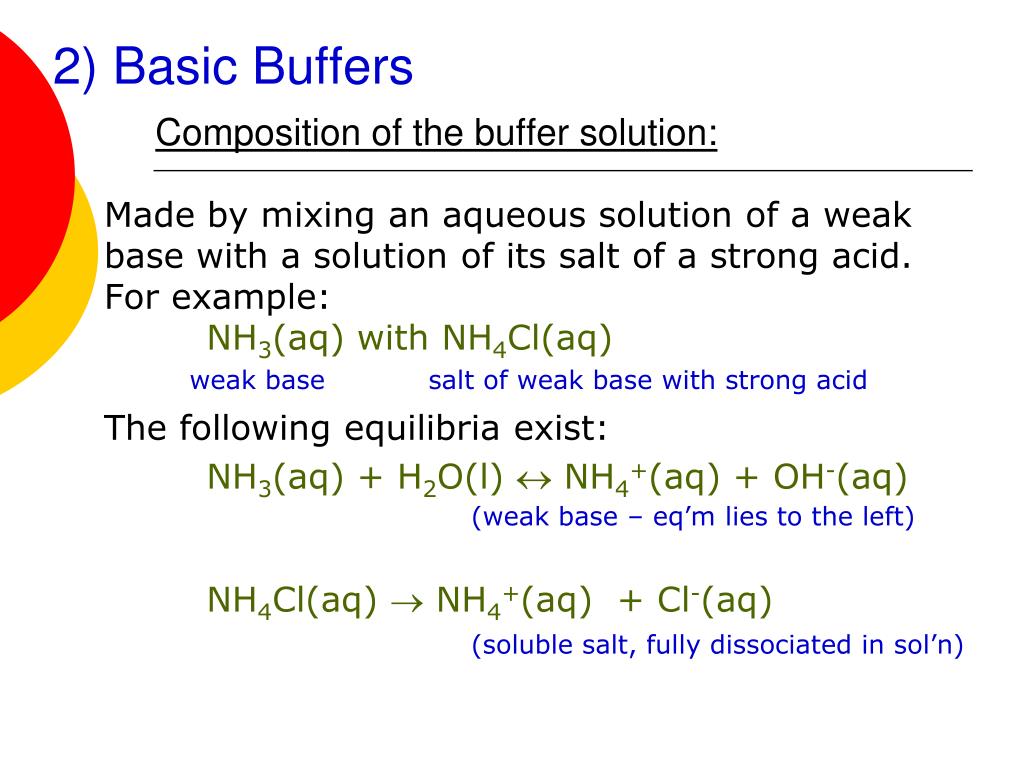
The aqueous solution of a salt of a strong acid and a strong base is neutral as neither the anion nor cation undergoes hydrolysis. HCl aq NaOH aq NaCl aq H 2 O l A solution which resists any change in its pH on dilution or on the addition of small amount of a strong acid or a strong alkali is called a buffer solution. Solutions that contain salts or hydrated metal ions have a pH that is determined by the extent of the hydrolysis of the ions in the solution.
The pH of the solutions may be calculated using familiar equilibrium techniques or it may be qualitatively determined to be acidic basic or neutral depending on the relative K a and K b of the ions involved. Summary of hydrolysis behavior. Salt of a strong acid and a strong base.
Neither cation nor anion hydrolyzes and the solution has a pH of 7 neutral 2. Salt of a strong acid and a weak base. Cation hydrolyzes form H ions solution has a pH of less than 7 acidic.
The extra OH- ions that came from the water hang around increasing the pH. So the answer to your question is yes the salt hydrolyzes. In fact the hydrolysis of the salt is what allows a buffer to be made.
You establish a ratio of concentrations of the salt and its acid to give a desired pH. Instead of adding tiny amounts of acid to affect a. If we dissolve NaF in water we get the following equilibrium.
The pH of the resulting solution can be determined if the of the fluoride ion is known. 200 g of sodium fluoride is dissolve in enough water to make 5000 mL of solution. Calculate the pH of the solution.
The of the fluoride ion is 14 10 11. Buffers are used to resist pH or pOH change in solutions. They contain a mixture of.
A strong pH or pOH will dissociate in water where weak bases and. Acids will not dissociate well. In buffers the pH is equal to the pKa log salt acid.
HYDROLYSIS OF SALTS AND pH OF BUFFER SOLUTIONS REPORT SHEET TABLE 75 Solution Ion Expected to Hydrolyze None Spectator Ion Naa C2H302 Nort 010 M Naci 010 M Naco 010 M NaCHO 010 M NHẠC 010 M ZnCI 010 M KAlSO 2n Zn24 So 4²- TABLE 76 Methyl Solution Red Bromothyol Blue Methyl Orange Phenol Red Phenoph- thalein Alizarin Yellow pH H OH 1 HO 883. When you have just a salt left with either or even both of its ions coming from a weak acidbase it is a case of SALT HYDROLYSIS. I mean to say no free acid or base is left.
Eg 100 mL 01 N CH3COOH 100 mL 01 N NaOH — 100 mL 005 N CH3COONa This need to be solved by SALT HYDROLYSIS. Hydrolysis and pH of Salt Solutions Calculating pH What is pH. Brief History pH is a measurement of acidity or alkalinity of a substance.
The scale ranges from 0-14 7 being neutral. The lower the number. The higher the number 7 the more.
The buffer H X N a X can be best used to maintain the p H in the range View Answer Calculate the p H nearest integer of 0. 0 1 0 M N a H C O 3 solution. Buffer Solutions and Hydrolysis.
Determination of the Heat of Reaction for Acid-Base Neutralization. Determination of the Keq for an Esterification Reaction. Multiple Step Synthesis Step I Bromination of Acetanilide.
Multiple Step Synthesis Step II Chlorination of 4-bromoacetanili. Hydrolysis of Salts and pH of Their Solutions. Hydrolysis of salt is the reaction of an anion or cation with water that results in the cleavage of the rm O H bond.
The phrase hydrolysis is derived from the words hydro which means water and lysis which means breaking. Hydrolysis of salts will be used to study the acid-base properties of dissolved ions in aqueous solutions. The approximate pH of these solutions will be determined using acid-base indicators.
A buffer solution will be prepared and its ability to moderate pH will be investigated alongside solutions that cannot function as buffers. Start studying solution ph and poh hydrolysis of salts and buffer solution. Learn vocabulary terms and more with flashcards games and other study tools.
The hydrolysis reaction above produces hydroxide ions thus the solution of the salt potassium nitrite will be basic. Buffers are solutions designed to maintain a relatively constant pH. The hydrolysis reaction above produces hydroxide ions thus the solution of the salt potassium nitrite will be basic.
Buffers are solutions designed to maintain a relatively constant pH when an acid or base is added. ABSTRACT The objective of experiment was to determine the value of pH of salt and solution by using hydrolysis and buffer method respectively. In general the hydrolysis of salt is a reaction in which the cation or anion or both of a salt react with water to produce acidity or alkalinity.
Both of these ions are able to give hydrolysis as we have seen in the previous examples. CH 3 COO-gives basic hydrolysis while acid hydrolysis is given by NH 4. The pH of a salt composed of weak acid and weak base will depend on the strength relationship of the acidic and basic components of the salt.
A salt that is derived from the reaction of a strong acid with a strong base forms a solution that has a pH of 7. An example is sodium chloride formed from the neutralization of HCl by NaOH. A solution of NaCl in water has no acidic or basic properties since neither ion is capable of hydrolyzing.