Sodium bicarbonate is preferable to NaOH in this process as it is a much weaker base. Exercise is repeated using acetic acid CH 3 COOH instead of benzoic acid and any similarities and differences are observed.
Furthermore what is the purpose of extraction.
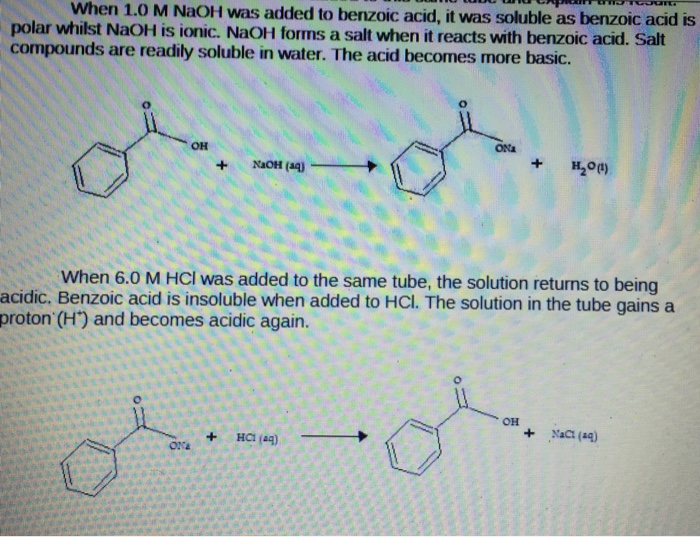
Benzoic acid naoh hcl. Benzoic acid is a weak acid and HCl is comparatively stronger acid however they wont react with one another as they each are acid. Nevertheless in presence of HCl the dissociation of benzoic acid will probably be gradual attributable to widespread ion impact. 1Why benzoic acid is soluble in NaOH.
2Why HCl is insoluble in titration of benzoic acid and NaOH. Benzoic is soluble in a solution of NaOH because the base forms the sodium salt with the acid to form sodium benzoate. Is Glucose soluble in HCl NaOH and NaHCO3 because its soluble in water.
Benzoic acid with hcl Reaction of benzoic acid with hcl. Benzoic acid with 6m hcl. In this experiment you will use two important organic separation protocols.
You will begin with a mixture of two different organic chemical products benzóico and m. Benzoic acid is a weak acid and HCl is relatively stronger acid but they will not react with each other as they both are acid. However in presence of HCl the dissociation of benzoic acid will be slow due to common ion effect.
Furthermore what happens to benzoic acid when heated. When benzoic acid is heated in presence of a strong dehydrating agent like P2O5 or H2SO4 it forms benzoic anhydride. Benzoic acid is a weak acid and HCl is relatively stronger acid but they will not react with each other as they both are acid.
Again no base will react with another baseHowever in presence of HCl the dissociation of benzoic acid will be slow due to common ion effect. Benzoic acid is as the name suggests an acid. It is essentially the carboxylic acid of toluene the simplest 6-membered ring carboxylic acid.
NaOH is a base. The H from the OH of the benzoic acid combines with the OH of NaOH to form water H2O. The Na cation joins the O- of the benzoic acid.
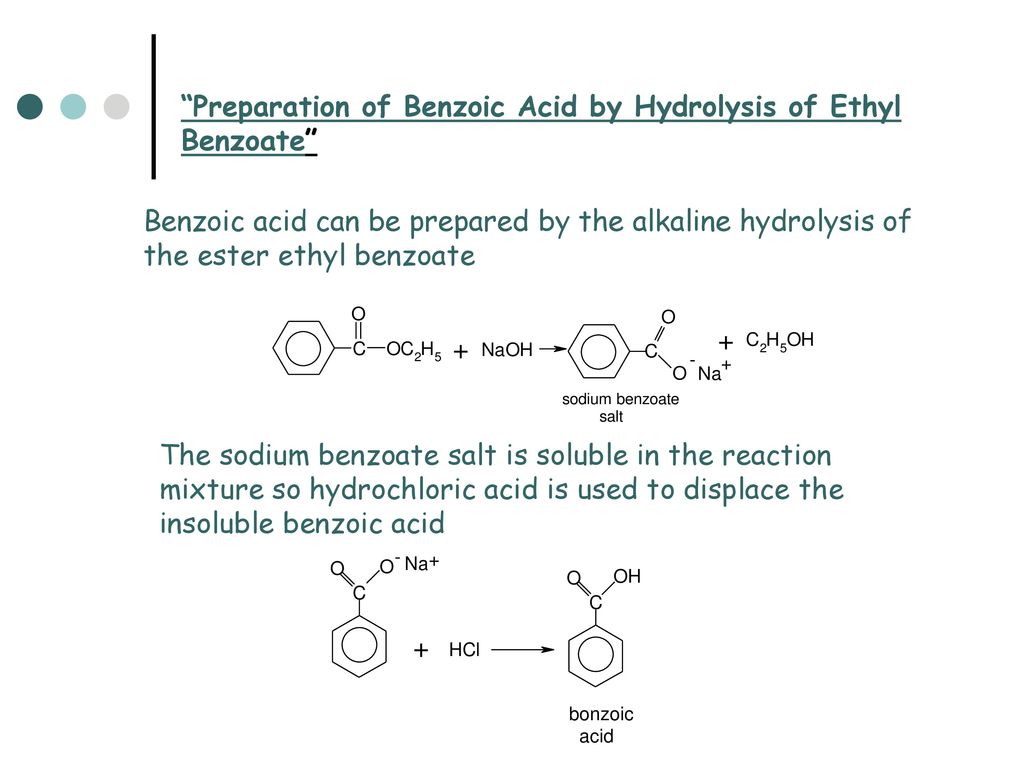
Thus the products - sodium benzoate and water. This solution was mixed with 10ml of 1M sodium hydroxide NaOH in a separatory funnel and then separated into an aqueous phase benzoate water and an organic phase naphthalene ethyl acetate. 3M hydrochloric acid HCl was added to the aqueous solution to acidify it re-creating a benzoic acid solution as a result.
Separated from the naphthalene and aniline figure 3. The ether layer containing benzoic acid can be evaporated off to provide the solid benzoic acid. Acidification of the Basic Solution naphthalene OH O benzoic acid NH2 aniline in ether NaOH H2O O O NH2 in ether in water Na O O in water Na HClaq OH O NaClin water add ether OH O.
Benzoic acid is insoluble in water. Soluble in 10 NaOH but when you add 60 M HCl to the same tube it becomes insoluble. Benzoic acid is insoluble in 10 M HCl b give a brief explanation that explains the solublity trend observed for benzoic ethyl-4-aminobenzoate.
You must explicity descrive the intermolecular forces involved. Benzoic -C6H5COOH C6H5COO 646 x 10-5 419 Oxalic 2 HC2O4-C 2O4 2-64 x 10-5 419 Hydrazoic -5HN3 N3-19 x 10 472 Citric 2 H2C6H5O7-HC 6H5O7 2-18 x 10-5 474 Acetic -5CH3COOH CH3COO-176 x 10 475 Propionic CH3CH2COOH CH3CH2COO-134 x 10-5 487 Pyridinium ion C5H4NH C 5H4N 56 x 10-6 525 Citric 3 HC6H5O7 2-C 6H5O7 3-40 x 10-6 540. Will NaOH Deprotonate benzoic acid.
Because benzoic acid is comparatively strong acid it can be deprotonated more easily than either 2-naphthol or naphthalene by a weak base. Aqueous sodium bicarbonate a weak acid was used to deprotonate the benzoic acid. An aqueos solution of 20 NaOH is a strong base that effectively deprotonated 2-naphthol.
A sample of benzoic acid contaminated with NaCl and sand is to be recrystallized. Solubility in water at 100 degrees Celsius in g100 mL 68 at 0 degrees Celsius in g100 mL 02. Solubility in water at 100.
Is benzoic acid soluble in. Benzoic Acid NaOH. Neutralization Reaction If playback doesnt begin shortly try restarting your device.
Videos you watch may be added to the TVs watch history and influence TV. Give the reaction equation and draw an arrow pushing mechanismof benzoic acid NaOH Give the reaction equation and draw anarrow pushing mechanism of sodium benzoate with HCl Give thereaction equation and draw an arrow pushing mechanismforo-toluidine hydrochloride salt NaOH. For the solubility of benzoic acid and of benzocaine ethyl p-aminobenzoate in water aqueous HCl and aqueous NaOH.
Now that you know some relevant acid-base chem you may know what should have happened. Thats not the question. Report what you actually observed Liquid-liquid extraction is one of the easiest and most common ways in which.
To the collected aqueous phase which is in the 100-mL beaker you will add enough 6 M HCl if you added about 10 mL of 10 NaOH 25 M you will need to add at least 5 mL of the 6 M HCl you cannot add too much so to be safe add 10 mL of your HCl solution to react with the sodium benzoate and convert it back into benzoic acid which is mostly insoluble in water. You will be able to. The acidic portion of benzoic acid is the carboxyl group and it reacts with a base to form a salt.
For example it reacts with sodium hydroxide NaOH to produce sodium benzoate C6H5COOH NaOH —– C6H5COO- Na H2O. Benzoic acid is a weak acid and HCl is relatively stronger acid but they will not react with each other as they both are acid. However in presence of HCl the dissociation of benzoic acid will be slow due to common ion effect.
The H from the OH of the benzoic acid combines with the OH of NaOH to form water H2O. What will happen if some amount of hydrochloric acid is added to a solution of. Ii To the solutions of benzoic acid and naphthol formed with NaOH solution HCl solution is added dropwise until solid cloudiness and precipitation is observed.
Exercise is repeated using acetic acid CH 3 COOH instead of benzoic acid and any similarities and differences are observed. A wash with sodium bicarbonate converts benzoic acid into its more water-soluble sodium benzoate form extracting it into the aqueous layer Figure 457. Sodium bicarbonate is preferable to NaOH in this process as it is a much weaker base.
Washing with NaOH could cause hydrolysis of the ester product. Furthermore what is the purpose of extraction. Benzoic acid is a weak acid and HCl is relatively stronger acid however they will not react v each other as castle both are acid.
However in existence of HCl the dissociation the benzoic acid will be slow-moving due to usual ion effect. The H from the five of the benzoic mountain combines with the oh of NaOH to kind water H2O. What will happen if part amount the hydrochloric mountain is included to a.
Is benzoic acid soluble in HCl. Benzoic acid was found to be soluble in water and 10 M NaOH however upon addition of 60 M HCl to this solution benzoic acid became insoluble. Benzoic acid was also insoluble in 10 M HCl.
Ethyl 4-amino benzoate was also soluble in 10 M HCl.